Introduction: Cytokine release syndrome (CRS) and immune effector cell (IEC)-associated neurotoxicity syndrome (ICANS) are common adverse effects following IEC therapy. Janus kinase (JAK) pathways are important for the signaling of several cytokines involved in CRS pathogenesis. Itacitinib is a potent, selective JAK1 inhibitor with broad anti-inflammatory activity. We describe preliminary results from the randomized, placebo-controlled portion of the study evaluating itacitinib 200 mg twice daily (bid) for the prevention of CRS.
Methods: This randomized, placebo-controlled portion of the phase 2 Study INCB 39110-211 (NCT04071366) enrolled patients ≥18 years old planning to receive axicabtagene ciloleucel for relapsed/refractory large B-cell lymphoma (rrLBCL) and follicular lymphoma (rrFL). Participants received oral itacitinib 200 mg bid beginning 3 days before axicabtagene ciloleucel infusion and continued itacitinib through Day 26. Protocol did not allow tocilizumab for treatment of grade 1 CRS unless CRS did not improve after 72 hours of supportive treatment. Patients who received tocilizumab for grade 1 CRS were not censored. Primary endpoint was incidence of CRS grade ≥2 by Day 14 using American Society for Transplantation and Cellular Therapy grading system. Secondary endpoints included incidence of ICANS by Day 28, duration of CRS and ICANS, and itacitinib safety.
Results: 47 patients with rrLBCL were randomized to itacitinib (n=23; median age, 66 [range: 29-80] years; male, 65%) or placebo arms (n=24; median age, 64 [range: 31-79] years; male, 71%). Median (range) number of prior regimens was 2 (1-6) and 1.5 (1-4), respectively. No rrFL patients enrolled in the study. Two (8%) patients in the itacitinib arm received platelet transfusions after Day 28; no transfusions were received in the placebo group.
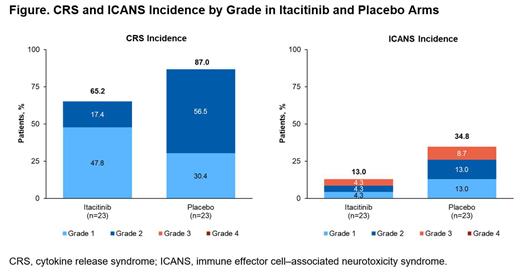
A total of 15 (65.2%) patients in the itacitinib arm developed CRS (grade 1, n=11 [47.8%]) compared with 20 (87.0%) patients in placebo arm (grade 1, n=7 [30.4%]; Figure). No hypotension or hypoxia was observed in patients with grade 1 CRS who received tocilizumab. Grade 2 CRS was experienced by 4 (17.4%) and 13 (56.5%) patients, respectively ( P=0.003; 95% CI: 0.14-0.65). There was no grade 3 or 4 CRS in either arm. Median (range) time to any-grade CRS onset was 2 (0-8) days and 3 (0-9) days, respectively; median (range) duration of grade 2 CRS was 2 (1-8) and 2 (1-5) days. Overall, tocilizumab was used for CRS treatment in 4 (17.4%) patients in the itacitinib arm and in 15 (65.2%) patients in the placebo arm, including 2 and 6 patients, respectively, who received tocilizumab for grade 1 CRS. ICANS occurred in 3 (13%; 95% CI: 3-34) patients in the itacitinib arm and 8 (34.8%; 95% CI: 16-57) patients in the placebo arm (Figure). ICANS grade ≥2 was reported in 2 (8.6%; 95% CI: 1-28) and 5 (21.7%; 95% CI: 8-44) patients, respectively. No grade 4 ICANS occurred in either arm. Median (range) time to any-grade ICANS onset was 5 (4-9) days and 6.5 (2-11) days, respectively; median (range) duration was 2 (2-11) and 3.5 (2-13) days.
Persistent grade 3 or 4 thrombocytopenia at Day 28 in the itacitinib arm was reported in 8 (38.1%; [grade 4: n=4]) patients; in the placebo arm in 4 (18.2%; [grade 4: n=0]) patients. Persistent grade 3 or 4 neutropenia at Day 28 in the itacitinib arm was reported in 7 (33.3%; [grade 4: n=3]) patients; in the placebo arm in 3 (13.6%; [grade 4: n=0]) patients. Infections grade ≥3 occurred before Day 28 in 2 (8.7%) patients in the itacitinib arm (urinary tract infection [UTI], n=2) and in 3 (12.5%) patients in the placebo arm (sepsis, n=2; UTI, n=1). There were no fatal adverse events. With a minimum follow-up of 28 days, lymphoma best overall response to axicabtagene ciloleucel showed no difference between the 2 arms.
Conclusions: Results from the randomized, placebo-controlled portion of Study INCB 39110-211 have shown prophylaxis treatment with itacitinib 200 mg bid to be well tolerated and resulted in a lower rate and grade of CRS and ICANS after lymphoma treatment with axicabtagene ciloleucel. Incidence of grade 3/4 neutropenia and thrombocytopenia not resolved at Day 28 was higher with itacitinib vs placebo. Rates of severe infections were comparable in both arms. Longer follow-up data on lymphoma response will be available upon presentation. Biomarker analyses comparing the effect of itacitinib on CAR-T cells expansion and function are ongoing.
Disclosures
Frigault:Kite, BMS, Novartis, Iovance: Consultancy; Kite, Arcellx, Novartis: Research Funding. Maziarz:Gamida: Research Funding; Orca Therapeutics: Research Funding; Athersys: Other: Patent holder; Kite: Consultancy; AlloVir: Consultancy, Research Funding; Novartis: Consultancy, Research Funding. Park:Genentech, Inc.: Research Funding; Incyte: Research Funding; Fate Therapeutics: Research Funding; Artiva Biotherapeutics: Consultancy, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Sobi: Consultancy, Research Funding; BeiGene: Consultancy; GC Cell: Membership on an entity's Board of Directors or advisory committees; Bright Pharmacetuicals: Consultancy; Servier: Consultancy, Research Funding; Kite: Consultancy; Affyimmune: Consultancy; Autolus Therapeutics: Research Funding; Allogene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Intella: Consultancy; Pfizer: Consultancy; Minerva Bio: Consultancy; Curocell: Consultancy; Takeda: Consultancy, Research Funding; Amgen: Consultancy; Be Biopharma: Consultancy. Lazaryan:Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Shah:Janssen: Consultancy; Epizyme: Consultancy; LOXO-Lilly: Consultancy, Other: Travel support; Tundra Therapeutics: Current holder of stock options in a privately-held company; BMS/Juno: Consultancy; Novartis: Consultancy; TG therapeutic: Consultancy; Umoja: Consultancy; Seattle Genetics: Consultancy; Gilead/Kite: Consultancy; Incyte: Consultancy; Abbvie: Consultancy; Lilly Oncology: Consultancy, Research Funding; Miltenyi Biotec: Consultancy, Other: Travel support, Research Funding. Svoboda:Seagen: Consultancy, Research Funding; Incyte: Consultancy, Research Funding; ADCT: Consultancy; Adaptive: Consultancy, Research Funding; Astra Zeneca: Consultancy, Research Funding; Atara: Consultancy; BMS: Consultancy, Research Funding; Genmab: Consultancy; Pharmacyclics: Consultancy, Research Funding; Merck: Research Funding. Reshef:Instil Bio: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Allogene: Consultancy, Honoraria; Gilead Sciences: Consultancy, Membership on an entity's Board of Directors or advisory committees; Synthekine: Consultancy, Membership on an entity's Board of Directors or advisory committees; TScan: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Orca: Consultancy, Membership on an entity's Board of Directors or advisory committees; Quell Biotherapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Capstan: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jasper: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bayer: Other: Witness role; Atara Biotherapeutics: Research Funding; Sanofi: Research Funding; Immatics: Research Funding; AbbVie: Research Funding; TCR2: Research Funding; Takeda: Research Funding; Gilead: Research Funding; CareDx: Research Funding; Synthekine: Research Funding; BMS: Research Funding; J&J: Research Funding; Genetech: Research Funding; Precision Biosciences: Research Funding. Burke:Incyte: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Lei:Incyte: Current holder of stock options in a privately-held company. Morariu-Zamfir:Incyte Corporation: Current Employment, Current equity holder in publicly-traded company. DiPersio:BiolineRx Ltd: Consultancy, Research Funding; Incyte: Consultancy, Research Funding; NeoImmune Tech: Consultancy; Macrogenics: Consultancy, Research Funding; Washington University: Current Employment; Amphivena Therapeutics: Research Funding; Vertex: Consultancy; Sun Pharma Ltd.: Membership on an entity's Board of Directors or advisory committees; hC Bioscience, Inc.: Membership on an entity's Board of Directors or advisory committees; RiverVest Venture Partners: Membership on an entity's Board of Directors or advisory committees; WUGEN: Other: Ownership Investment, Patents & Royalties; Magenta: Other: Ownership Investment, Patents & Royalties.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal